HIPAA compliance testing in Software quality assurance (SQA) refers to the process of ensuring that healthcare software meets the stringent standards set by the HIPAA privacy rules and HIPAA security rules. This testing focuses on verifying that software applications securely handle Protected Health Information (PHI), following HIPAA regulations for confidentiality, integrity, and accessibility. QA teams perform a variety of tests, including security testing, data encryption, and access control checks, to ensure that the quality assurance software adheres to HIPAA laws. The goal is to protect patient data from unauthorized access, ensuring legal compliance and reducing the risk of penalties.
What’s next? Keep scrolling to find out:
🚀 HIPAA Regulations: Key HIPAA laws and their impact on healthcare software.
🚀 QA’s Role in Compliance: How QA ensures HIPAA standards are met.
🚀 Testing Challenges: Tips for overcoming common HIPAA testing hurdles.
🚀 Best Practices: Essential tips for effective HIPAA compliance testing.
🚀 Compliance Testing Tools: Tools to streamline your testing process.
Understanding HIPAA and Its Impact on QA
HIPAA (Health Insurance Portability and Accountability Act) is a set of regulations designed to protect sensitive patient information. It mandates healthcare organizations to implement strict measures to ensure the confidentiality, integrity, and availability of Protected Health Information (PHI). For Software quality assurance testing services, understanding HIPAA regulations is critical to ensuring software meets compliance requirements. QA teams must test healthcare management software to verify that it adheres to the HIPAA privacy rule and HIPAA security rules, ensuring data protection and preventing unauthorized access. This understanding shapes testing strategies and helps avoid penalties while safeguarding patient data.

What Is HIPAA Compliance Testing?
HIPAA compliance testing is the process of evaluating healthcare software to ensure it meets the requirements outlined in the HIPAA law, which is designed to protect Protected Health Information (PHI). This testing is crucial for healthcare applications to confirm they adhere to the HIPAA privacy rule and HIPAA security rule.
Key Components of HIPAA Compliance Testing:
- Privacy and Security Rules: Verifying that the software complies with both the HIPAA privacy rule (which ensures the confidentiality of patient information) and the HIPAA security rules (which focus on safeguarding data from breaches or unauthorized access).
- Testing Areas:
- Security Testing: Ensures that data is protected from threats through encryption, firewalls, and other security measures.

- Access Control: Verifies that only authorized users can access sensitive data, using role-based access controls and authentication mechanisms.
- Data Integrity Testing: Ensures ePHI privacy is secure, accurate, and reliable during transmission or storage, using secure encryption and preventing tampering with ePHI.
- Audit and Reporting: Verifies that the system generates audit logs and comprehensive reports for compliance reviews.
Importance of HIPAA Compliance Testing:
- Avoiding Penalties: Ensures healthcare organizations prevent fines and penalties by meeting regulatory standards.
- Data Protection: Safeguards patient data from unauthorized access, ensuring confidentiality and security.
- Trust and Reliability: Helps build trust within the healthcare sector by ensuring that systems are secure and compliant.
Key HIPAA Rules Relevant to QA Professionals
For quality assurance services in healthcare software, understanding the HIPAA rules is essential for ensuring that applications comply with the law’s standards for data privacy and security. The following are the key HIPAA regulations that QA teams must be aware of:

1. HIPAA Privacy Rule
The HIPAA privacy rule establishes national standards for the protection of PHI (Protected Health Information). It outlines how personal health information should be handled, stored, and transmitted, ensuring that only authorized individuals have access to sensitive data. QA teams must ensure that software applications implement proper privacy controls, like data masking and encryption, to safeguard patient information.
2. HIPAA Security Rule
The HIPAA security rule focuses on the protection of electronic PHI (ePHI). It mandates the implementation of security measures like encryption, firewalls, and authentication to prevent unauthorized access. QA professionals are responsible for testing these security mechanisms to ensure they are functioning as intended and that ePHI is secure from potential breaches.
3. HIPAA Breach Notification Rule
The HIPAA Breach Notification Rule requires healthcare organizations to notify patients and the Department of Health and Human Services (HHS) about data breaches. QA teams ensure that software complies with security protocols, logs breaches, and meets regulatory compliance through compliance testing services, risk analysis, and automated testing tools.
4. HIPAA Enforcement Rule
The HIPAA enforcement rule sets the penalties for non-compliance, including financial penalties. QA professionals must ensure that systems are in place to track and document compliance with HIPAA, which can be audited to avoid penalties in case of violations.
Importance of HIPAA Compliance in Software Testing
HIPAA compliance in software testing services is crucial for protecting PHI in healthcare applications. QA teams ensure data privacy and prevent unauthorized access using thorough testing software methods.
1. Protecting Patient Data
HIPAA compliance ensures that patient data is securely HIPAA compliance ensures that patient data is securely handled.

2. Avoiding Legal and Financial Penalties
QA professionals performing software testing service assessments help identify gaps in security and performance measures to ensure compliance and avoid penalties.
3. Building Trust
Compliance with HIPAA strengthens patient trust. QA testing ensures that healthcare analytics software adheres to privacy and security standards, instilling confidence in both patients and healthcare providers.
4. Meeting Regulatory Requirements
QA professionals validate that software meets HIPAA privacy and security rules, ensuring proper encryption, authentication, and data handling as required by law using software functional testing techniques.
5. Protecting Against Cybersecurity Risks
HIPAA compliance testing helps identify vulnerabilities in software to protect against breaches and cyberattacks, safeguarding ePHI from potential threats.
Types of Testing Involved in HIPAA Compliance
HIPAA compliance involves several layers of testing that ensure healthcare software protects sensitive data and meets all regulatory requirements. QA professionals play a vital role by conducting specific types of testing that focus on data security, privacy, and data protection. Below are the key testing types necessary for achieving HIPAA compliance.
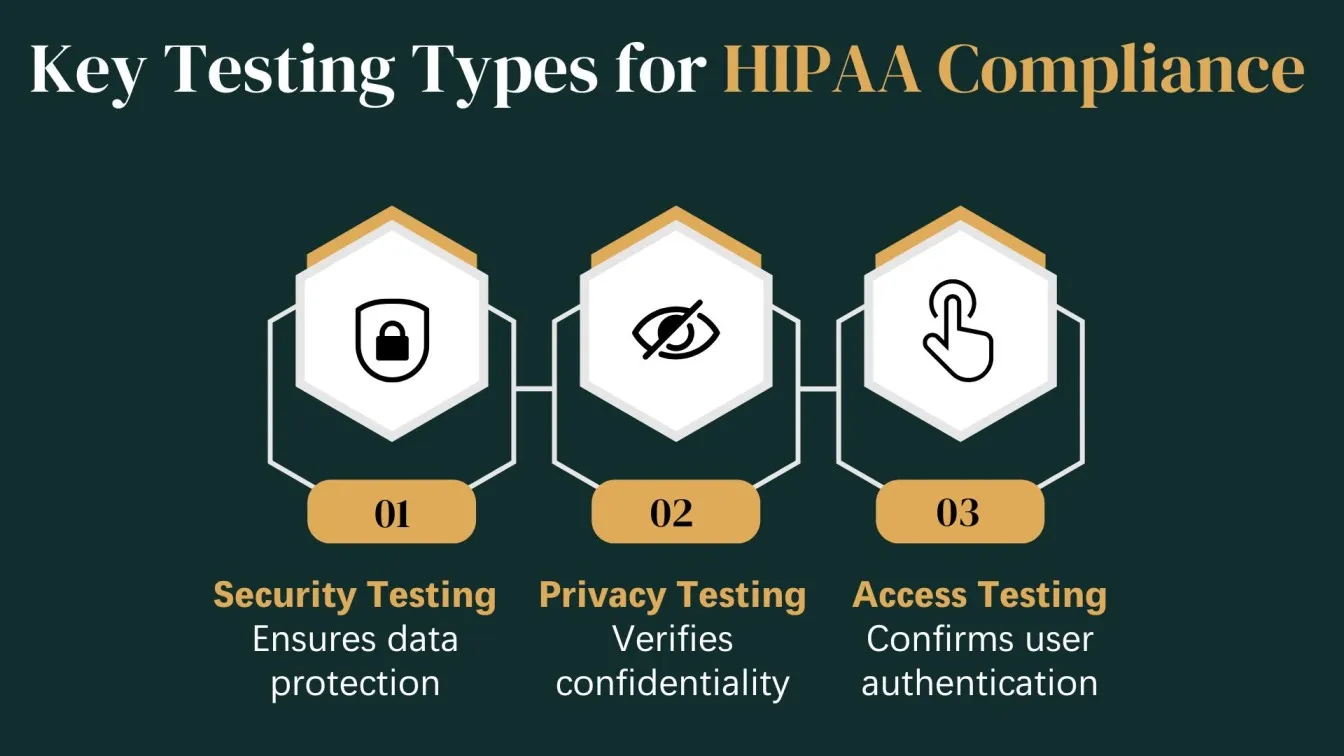
Security Testing
Security testing is essential to verify that a healthcare system can safeguard electronic Protected Health Information (ePHI) from breaches and cyber threats. This testing ensures compliance with the HIPAA Security Rule and evaluates the system's defense mechanisms.
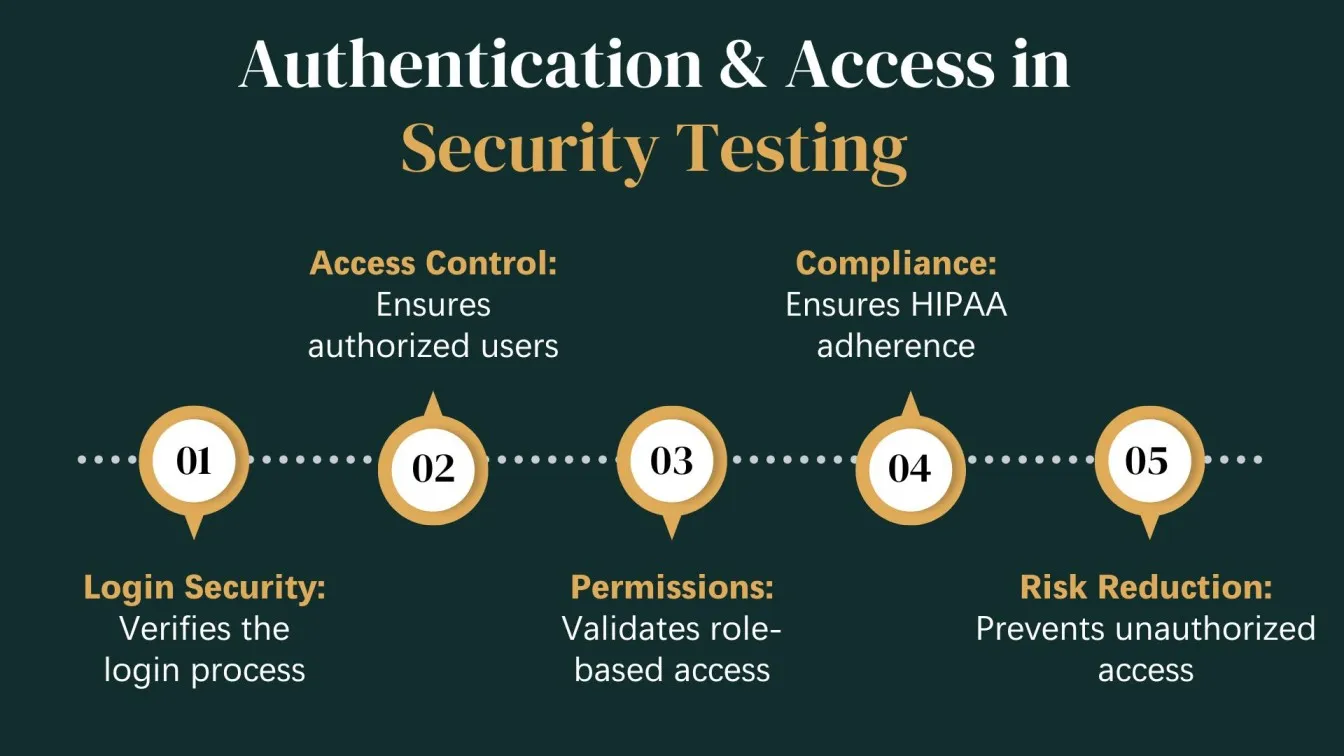
Key Focus Areas:
- Authentication & Authorization: Verifying secure login processes, user roles, and permissions.
- Data Encryption: Ensuring data is encrypted both at rest and in transit.
- Vulnerability Scanning: Detecting security gaps that could expose PHI.
- Intrusion Detection: Testing firewalls, antivirus tools, and monitoring systems.
Objective:
To ensure healthcare applications are secure against unauthorized access, tampering, and cyberattacks, helping organizations avoid costly breaches and legal penalties.
Privacy and Access Control Testing
This testing ensures that only authorized individuals can view or handle PHI, aligning with the HIPAA Privacy Rule. It evaluates how data is accessed, shared, and protected within the application.
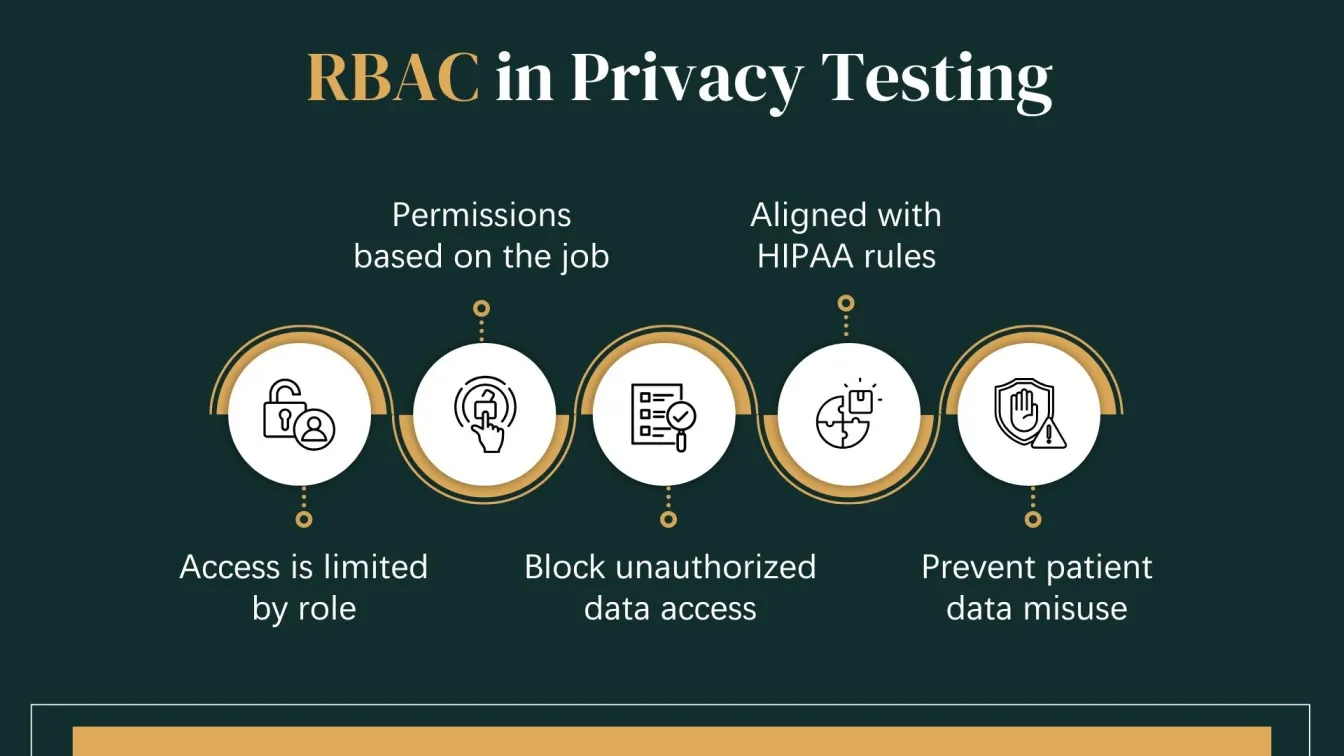
Key Focus Areas:
- Role-Based Access Control (RBAC): Verifying that access is limited based on job responsibilities.
- Session Management: Ensuring session times out and cannot be hijacked.
- Audit Trails: Confirming all access to PHI is logged for future auditing.
- Consent Management: Ensuring patient consent is respected in data sharing.
Objective:
To validate that the healthcare software respects privacy policies and prevents unauthorized use or disclosure of patient information.
Data Integrity and Backup Testing
Maintaining the integrity and availability of data is crucial under HIPAA regulations. This testing ensures that healthcare data remains consistent, unaltered, and recoverable.
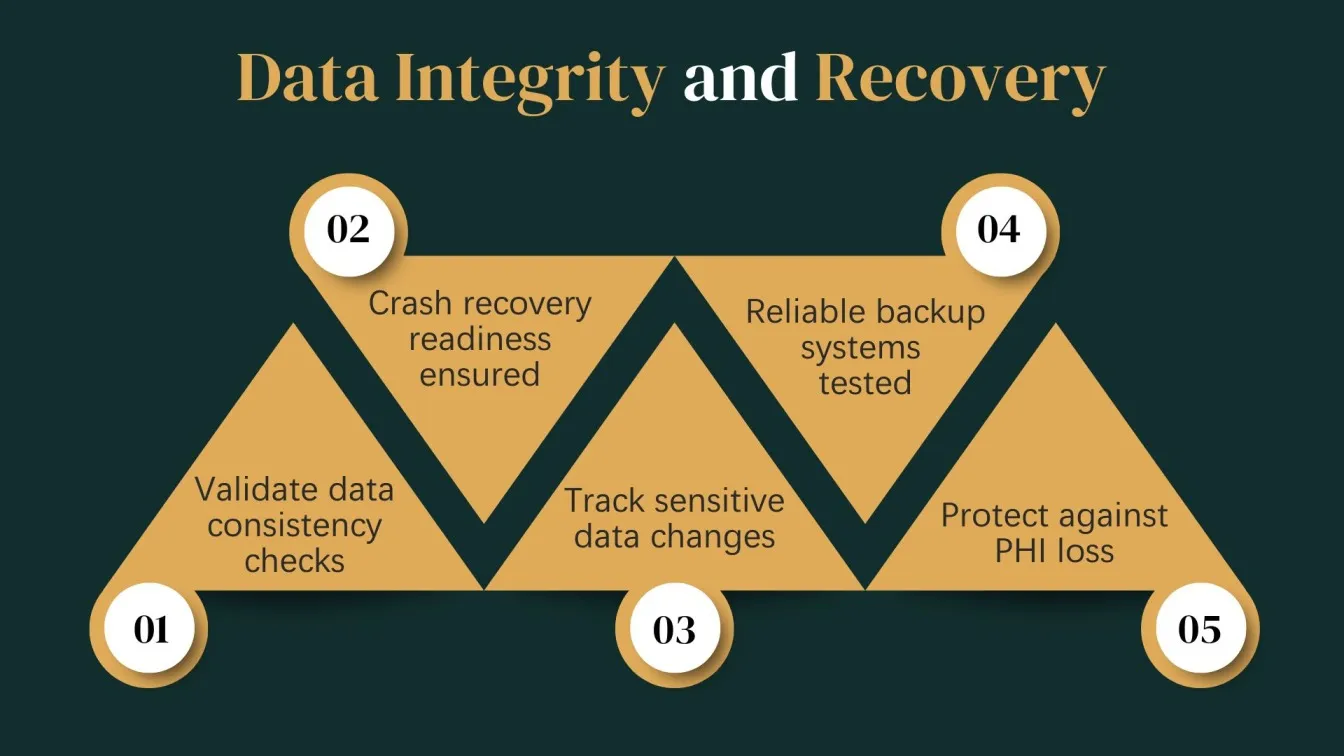
Key Focus Areas:
- Data Validation: Verifying that no information is lost or modified during transactions.
- Backup & Recovery: Testing automated and manual backup systems for reliability.
- Disaster Recovery Plans: Ensuring systems can recover data after a crash or breach.
- Version Control: Tracking changes made to data over time.
Objective:
To confirm that PHI can be reliably stored and retrieved without loss or corruption, even during emergencies, system failures, or attacks.
What is the Role of QA Teams in Ensuring HIPAA Compliance
Quality Assurance testing teams play a critical role in ensuring that healthcare software adheres to HIPAA compliance standards. QA ensures the app meets HIPAA security and privacy standards using application security testing tools.
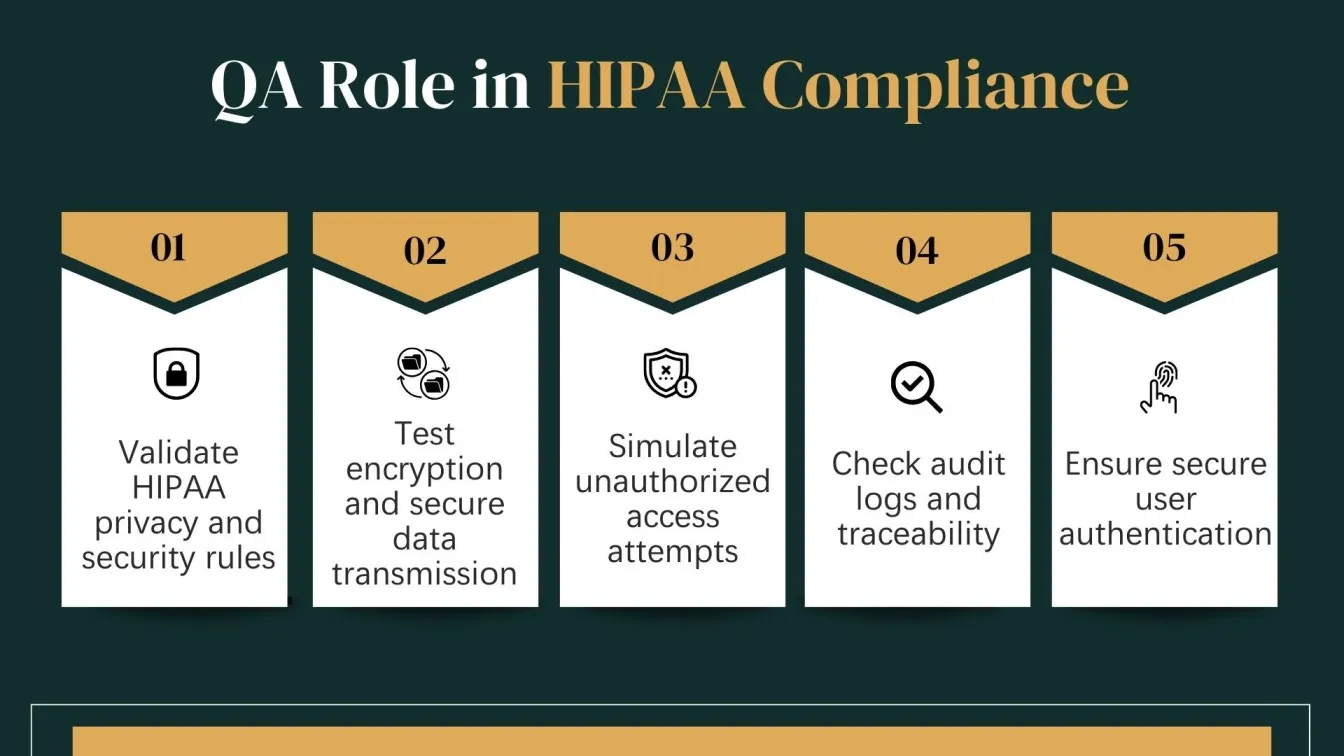
QA professionals must validate that healthcare software development services protect Protected Health Information (PHI) through thorough testing procedures such as security testing tools, privacy rule validation, and data integrity checks. They design compliance testing plans based on HIPAA guidelines, simulate real-world scenarios, and assess how systems respond to unauthorized access attempts or security breaches.
In addition, QA teams ensure that access controls, audit logs, user authentication, and encryption standards are implemented correctly and function reliably under all conditions. Their test documentation also serves as crucial evidence during compliance audits.
By aligning their test strategies with HIPAA rules and collaborating with the development team, legal teams, and security experts, QA professionals become a key line of defense in achieving and maintaining software compliance. Their work directly contributes to building trustworthy, HIPAA-compliant healthcare software solutions that keep patient data safe and ensure regulatory compliance.
Common Challenges Faced During HIPAA Compliance Testing
HIPAA compliance testing introduces unique challenges for QA professionals, especially when dealing with complex healthcare software systems. Unlike general software testing, HIPAA testing demands a deep understanding of security, privacy, and regulatory requirements.
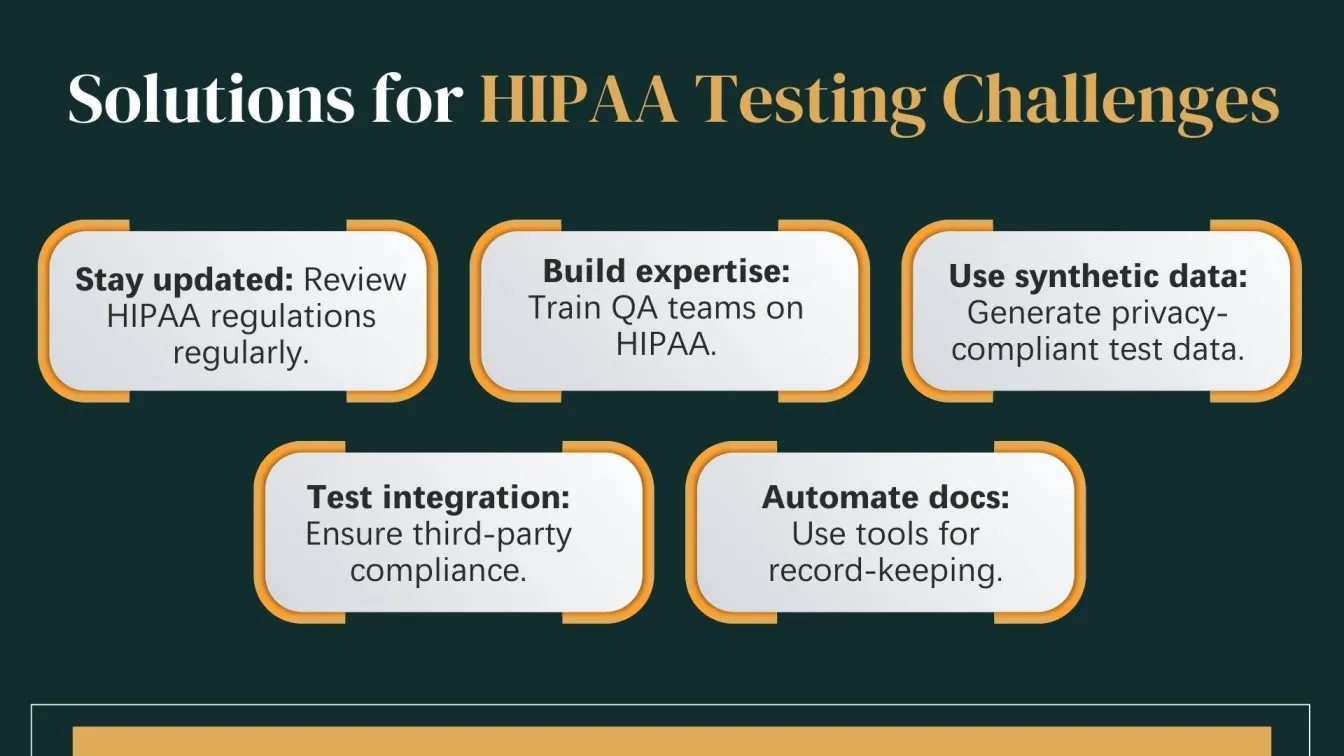
1. Evolving Regulations:
HIPAA laws are frequently updated, making it difficult to maintain test cases that reflect the latest compliance requirements.
2. Lack of Domain Expertise:
QA testers may lack specific knowledge about HIPAA rules, such as the Privacy Rule or Security Rule, leading to oversight in critical testing areas.
3. Data Sensitivity:
Using real patient data is restricted. Health plan systems must generate valid test data during the development cycle without breaching PHI laws.
4. Integration:
Healthcare systems often link with third-party apps. Ensuring compliance across platforms needs rigorous testing and coordination.
5. Audit-Ready Documentation:
Maintaining audit-ready records of tests and outcomes takes effort. Automation testing supports this while ensuring ongoing compliance.
Best Practices for HIPAA Compliance Testing
To ensure HIPAA compliance, QA teams must adhere to best practices that focus on privacy, security, and data integrity. Key practices include creating comprehensive test cases aligned with HIPAA regulations, ensuring encryption of PHI, and verifying access controls and user permissions. Regular security testing, such as vulnerability scanning and penetration testing, is essential. Additionally, QA teams should maintain detailed documentation for audit purposes and conduct periodic reviews to stay updated with evolving HIPAA laws. Collaboration with security and legal teams also ensures thorough compliance validation.

Create Test Cases Aligned with HIPAA Requirements
In the healthcare industry, QA teams must design test cases that align with HIPAA's privacy and security standards. These test cases should cover critical aspects like PHI protection, user access controls, and breach detection, ensuring the system meets all compliance requirements. Sanity testing is essential to confirm system stability and security during these processes. Each test scenario should reflect real-world healthcare scenarios to guarantee compliance with HIPAA regulations and audit control standards.
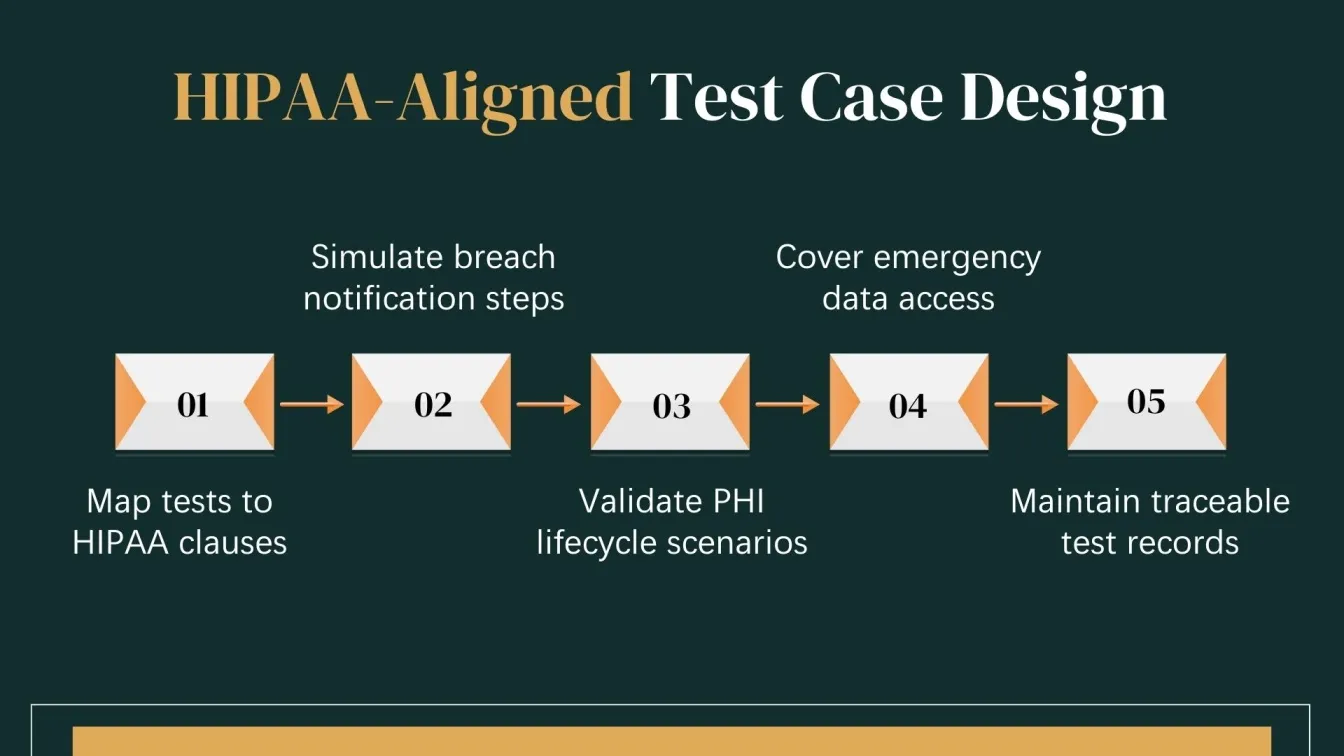
Key Areas to Focus on:
- PHI Lifecycle Testing:
The testing team must design cases covering the full lifecycle of PHI, including creation, storage, transmission, access, and deletion, ensuring compliance at every stage. - Breach Notification Validation:
Simulate breach scenarios to confirm that breach notifications are triggered promptly, ensuring the system follows HIPAA's breach reporting rules. - Comprehensive Documentation:
Testing teams should ensure thorough documentation of test cases, results, and procedures, providing a clear compliance trail for audit control and HIPAA adherence.
Maintain Detailed Test Documentation for Audits
Maintaining detailed test documentation is vital for demonstrating HIPAA compliance during regulatory audits. QA professionals must ensure that all test cases, results, and compliance checks are meticulously documented to provide a clear audit trail. These records not only help in verifying compliance software testing but also serve as evidence in the event of a security breach or legal dispute.
Key Aspects of Test Documentation:
- Test Case Logs: Document each test scenario, expected results, and outcomes to ensure traceability and accountability. Ensure logs are compliant with compliance testing regulations.
- Issue Tracking: Record any security threats or privacy violations encountered during testing, along with the resolution steps taken. Track risks based on their risk level and update regularly.
- Audit Reports: Generate clear, detailed reports summarizing testing efforts, compliance verification, and findings. Reports should include vulnerability assessments, encryption algorithms, and other security requirements to make them easy to present during audits.
Conclusion: Empowering QA Teams for HIPAA-Compliant Software
Ensuring HIPAA compliance in healthcare apps requires a structured development cycle, with QA teams working alongside developers to integrate robust security measures. By focusing on automation testing, they ensure rigorous testing of features like two-factor authentication and secure access controls. QA teams validate that users with access to patient data follow strict protocols to maintain patient privacy and data security.
Using artificial intelligence, they enhance the detection of vulnerabilities and automate compliance checks, ensuring patient data security is never compromised. Regular assessments help maintain continuous alignment with HIPAA regulations, supporting healthcare service providers in safeguarding sensitive health plan information. With their expertise in mobile device testing, QA teams play a crucial role in ensuring that healthcare apps meet the highest standards of compliance, protecting patient privacy while building trust in healthcare systems.
Furthermore, QA teams ensure that healthcare operations comply with regulations by facilitating Frugal Testing is a leading SaaS application testing company known for its AI-driven test automation services. Among the services offered by Frugal Testing are cloud-based test automation services that help businesses improve testing efficiency, ensure software reliability, and achieve cost-effective, high-quality product delivery.
People also Ask
Can HIPAA Compliance Testing Be Automated?
Yes, automation helps check access, encryption, and policy enforcement efficiently, saving time.
Are there any HIPAA-compliant AI tools?
Yes, several AI tools support secure, compliant healthcare data processing, ensuring privacy.
What type of risk assessment is required by HIPAA?
HIPAA requires a thorough Security Risk Assessment for all systems, identifying vulnerabilities.
How often are HIPAA audits done?
Audits occur randomly or yearly to ensure ongoing HIPAA compliance, ensuring readiness.
How much does HIPAA certification cost?
Costs vary widely, typically between $1,000 to $20,000 overall, depending on factors.





%201.webp)

